- Share
- Share on Facebook
- Share on X
- Share on LinkedIn
1. Preventing PTP opening in β-cells.
In continuity of work in progress, we wish to develop strategies to prevent PTP opening in the Langerhans islet β-cells. These cells have the particularity to suffer of both, an excess or a deficit of glucose. In both cases, this triggers PTP opening and cell death in β-cells.
2. Molecular mechanisms linking PTP to apoptosis.
Even if it is now well settled that prolonged opening of PTP triggers cell death, the molecular mechanisms by which PTP opening leads to apoptosis by the release of mitochondrial pro-apoptotic proteins are poorly understood. The classical model by which PTP opening leads to swelling of mitochondria and thus a mechanical rupture of the outer mitochondrial membrane has been established with isolated mitochondria; it is probably not true in intact cells. We test novel hypothesis of PTP signaling.
3. Diabete cell therapy
Insulin dependent diabetes mellitus results from a loss in the physiologic function of insulin secretion due to immune reaction or metabolic toxicity driven against islets (the anatomical functional unity containing insulin secreting cells).
With 424 million people suffering from diabetes worldwide in 2017, diabetes emerges as a major public health problem and to date, diabetes remains an incurable disease. The medical treatment of diabetes has a considerable impact on the quality of life of patients and although exogenous insulin therapy is effective at preventing acute metabolic decompensation and is life-saving for insulin dependent diabetic patients, 70% of diabetic patients are inadequately controlled and diabetes remains one of the leading cause of cardiovascular disease (CVD), blindness, kidney failure or lower limb amputation heavily impacting patients’ quality of life and making diabetes a disease associated with high medical costs. Regenerative medicine with islet transplantation, a cell therapy for diabetes, has emerged for a decade as a promising effective treatment to restore good glycaemic control in insulin independent diabetic patients with severe form of diabetes. Unfortunately, the extension of this existing cell therapy to the general population of type 1 diabetic patients is limited by 1) life-long administration of immunosuppressive drugs, which is frequently associated with severe side effects 2) islet scarcity 3) loss of islet graft functionality over time.
In our group, we aim to develop strategies including innovative biomaterials and constructs that will allow to replace the deficient native islets and to sustain long-term viability and functionality of grafted islet to restore normal and physiological endogenous insulin secretion in diabetic subjects and to cure diabetes.
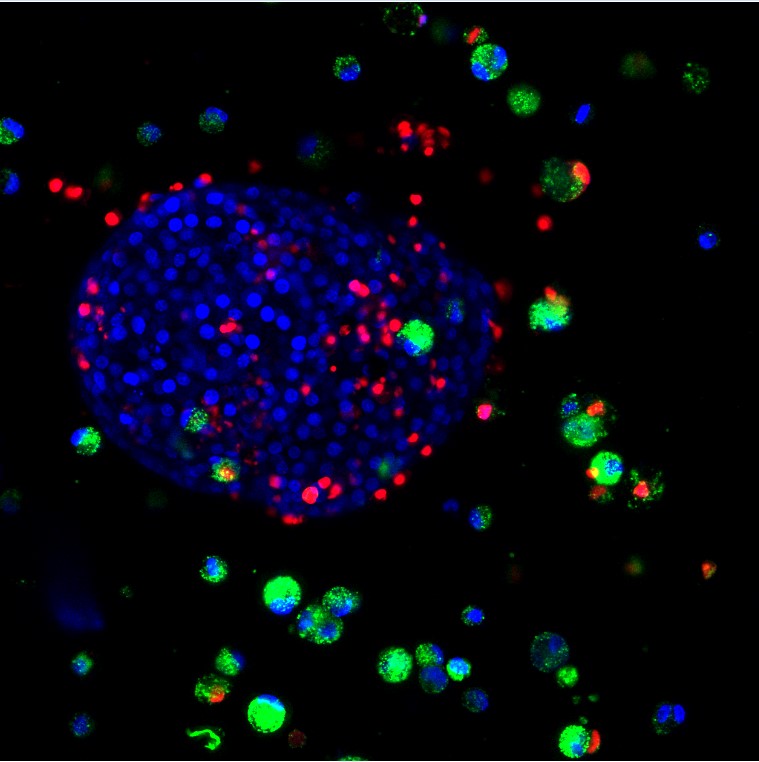
Our research is focused on the development and evaluation of innovative devices aiming at enhancing current diabetes cell therapy. The BIOCAPAN project, developed by the BIOCAPAN collaborative consortium, is one of the projects developed in our group: this project aims at proposing an immunosuppressive-free cell therapy for the treatment of diabetes and allowed the design of a patented microcapsules that demonstrated enhanced performance in vitro and in vitro compared to standard microcapsules.
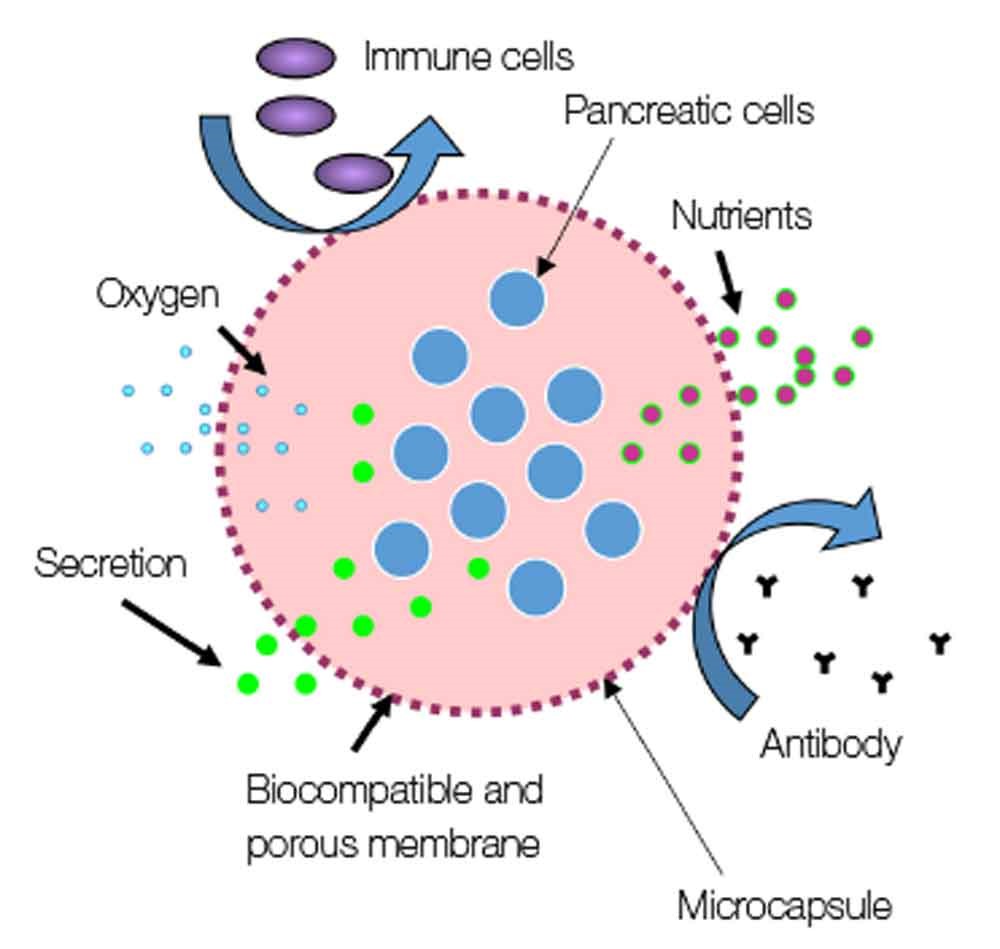
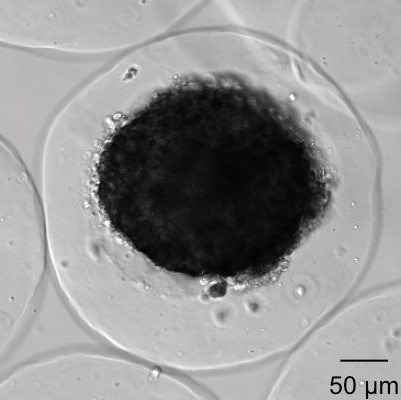
Details of the project can be found on the BIOCAPAN website. Our group is involved in an organ-on-chip project aiming at developing a pancreas on chip with for pharmaceutical regenerative medicine application (read more).
Coordinator

Sandrine LABLANCHE, MCUPH
sandrine.lablanche univ-grenoble-alpes.fr (sandrine[dot]lablanche[at]univ-grenoble-alpes[dot]fr)
univ-grenoble-alpes.fr (sandrine[dot]lablanche[at]univ-grenoble-alpes[dot]fr)
Phone number: +33 (0) 4 76 63 53 98
Office number: 205.2
- Share
- Share on Facebook
- Share on X
- Share on LinkedIn